Write the electron configuration for the ion. You can see from this example that the MF is a multiple of the EF and represents the actual number of each type of atom that makes up the compound.

6 maths and consist of step by step solutions of all questions with answers.
Chemistry ef and mf worksheet answers. Chemistry Ef And Mf Worksheet Answers - Worksheet List. Chemistry Ef And Mf Worksheet Answers - Promotiontablecovers. Chemistry Ef And Mf Worksheet Answers - Promotiontablecovers.
To determine the molecular formula you set the EF mass taken X times and set it equal to the Molecular Weight of the compound. You can see from this example that the MF is a multiple of the EF and represents the actual number of each type of atom that makes up the compound. Octane a compound of hydrogen and carbon has a molar mass of 11426 gmol.
If one mole of the compound contains 1817 g of hydrogen what is its molecular formula. Find the molecular formula of a compound that contains 3045 nitrogen and 6955 oxygen. The molar mass of the compound is 9202 g.
Answers are in parentheses at the end of some problems. What is the empirical formula for a compound containing only carbon and hydrogen if it is known to contain 842 carbon. Find the empirical formula of a compound that contains 158 g of Al 281 g of S and 561 g of oxygen.
EMPIRICAL FORMULA MOLECULAR FORMULA WORKSHEET. Find the molecular formula of a compound that has a molar mass of 26 gmol and has an empirical formula of CH. EF is CH mass 12 1 13 gmol.
MF nEF n molar mass empirical mass 90 30 3. So MF C3H6O3. UNIT 3 WORKSHEET 1.
Determine the MF mass EF mass ratio for each substance and enter it into Column B of the table in Model 2. Be sure that your entire group agrees on the values. Look at the Information in Column C and Column D that is given for ethyne and ethene and write a complete.
Honors Chemistry Name _____ HW Hydrates Percent Composition EFMF Date _____ Complete these problems on a separate sheet of paper. Determine the percent Oxygen in Potassium Perchlorate. KClO4 Molar Mass is 138547 g KClO4 4 moles of O in KClO4 Molar mass of O is 15999.
Percent Composition And Empirical Formula Worksheet 7-3 PC EF MFpdf Sections 15 02 and 21. Review for your exam on Chapter 7. Answers to the review sheets are at the bottom of this page.
Mole Mega Worksheet Masterpdf and Molecular Formula Worksheet Masterpdf. Department of Chemistry University of Texas at Austin Tera 1012 Giga 109 Mega 106 Kilo 103 Hecto 102 Deca 101 deci 10-1 centi 10-2 milli 10-3. Micro 10-6 nano 10-9 pico 10-12 fempto 10-19 More Practice.
Energy Frequency Wavelength and the Photoelectric Effect. There are two equations you should know. Chapter 13 Chemistry Worksheet Answers.
Für diese Seite sind keine Informationen verfügbarWeitere Informationen. Class 6 maths chapter-13 Symmetry Questions worksheet with solutions. 6 maths and consist of step by step solutions of all questions with answers.
21122018 Blog. Download answers to the practice and summary questions in your AQA GCSE Sciences 91 Biology Chemistry and Physics Student Books. We use cookies to enhance your experience on our website.
By continuing to use our website you are agreeing to our use of cookies. 0737 2 O. 0369 1 EF.
Rb 2 O 0369 0369 8. A compound has an empirical formula of C 2 H 8 N and a molar mass of 46 grams per mole. What is the molecular formula n 1 MF C 2 H 8 N 18061023mol 1 Mole 440gM 132g 23 mol 1 Mole 025 M.
View Practice Test Answer Key from SCIENCE Chemistry at Brunswick High School Brunswick OH. Ll3 L2 Practice Test on Moles EF MF- Comp Short Answers. Show all work putting final answers to the.
Oj ujbm np mf tt j oj ujbm uf nq fsbu vs f gjo bm qs f ttv sf g jo bm wp mv nf gjo bm np mft g jo bm uf nq fsbu vs f 17 o5 17 o5 17 17 7 5 7 5 ojujbmwpmvnf gjobmwpmvnf ojujbmnpmft gjobmnpmft fbuhbjofepsmptu nbtt dibohfjo ufnqfsbuvsf 2n d5 q fouibmqz pgqspevdut fouibmqz pgsfbdubout g p g p q sp ev dut s fbdu bo ut 530. Worksheets and contact me for help. And the use of stoichiometric principles.
Answers to calculations must demonstrate correct units and appropriate use of significant figures. Tutorial on Sig figs. EF and MF Calculations.
More EF and MF calculations. Even MORE EF and MF Questions. Mole calculations W1 T2 Mole.
Honors Chemistry Molecular Formula Worksheet From EF and MM of MF 1. State the empirical formula for each of the following compounds with molecular formulas. A C 4 H 8 x 4 b C 2 H 6 O 2 c N 2 O 5 x 1 d Ba 3 PO 4 2 e Te 4 I 16 a CH 2 b _____ c N 2 O 5.
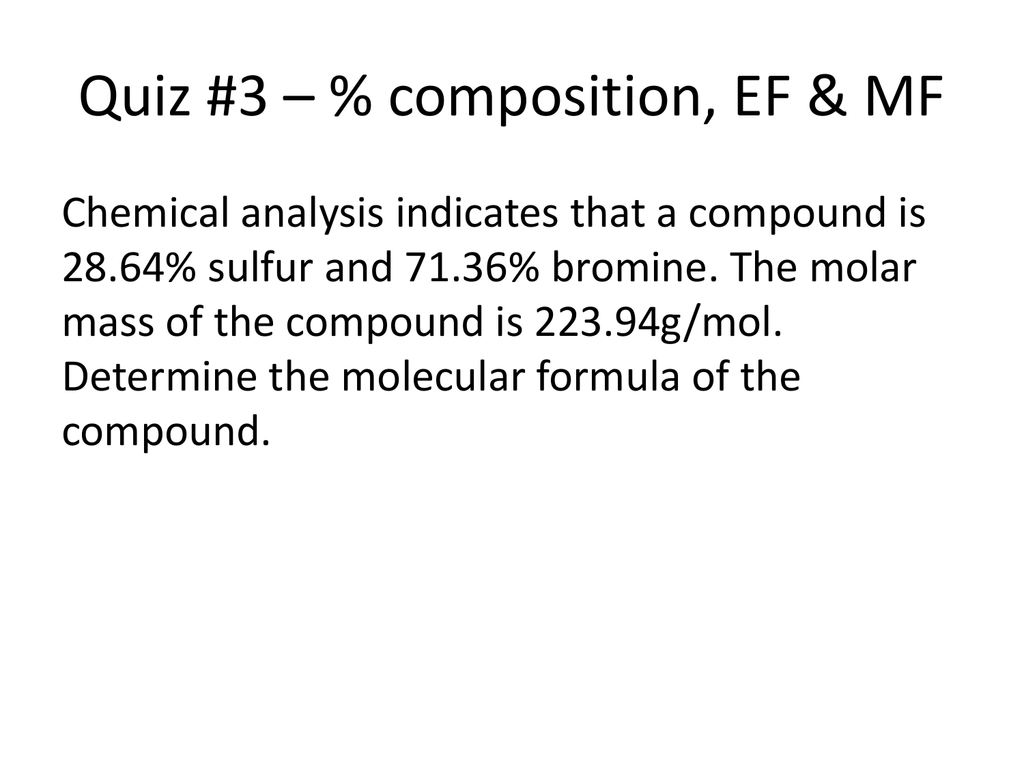
Quiz Chapter 3 Due. 092721 Name After each problem show your work or email copy of your readable hand written work. A 17000 g sample of an unidentified compound contains 2984 g sodium 6749 g chromium and 7267 g oxygen.
What is the compounds empirical formula. A compound containing 59265 H and 940735 0 has a molar mass of 3401468 gmol. Determine the empirical and.
CLASSIFYING MATTER -PowerPoint topics. Matter Pure Substances Mixtures. Student Handout for notes.
PHASE CHANGES ENERGY - PowerPoint topics. Energy Kinetic Molecular Theory phase changes. Student Handout for notes.
Evidence of a Chemical Change. Study Guide for unit. MgO Demo Discussion of formal lab reports.
MgO Formal Lab report due Wed Apr 27 Submission Link. Community Clean-up April 21 2016. Molecular Formula Formula of a Hydrate Worksheet.
Quiz - EF MF Hydrates Stoichiometry April 26 2016. CliffsAP 5 Chemistry Practice Exams by Gary S. Consultant Jerry Bobrow PhD.
01_770264 ffirsqxd 5306 223 PM Page iii. Loe VLIence sLwli e. Write the electron configuration for the ion.
How would the radius of the ion compare to the radius of the neutral atom. Use Coulombs law to justify your response. E SccYne level liqCVt4–C or czAracžLJD-j loq kww bchveen CUI VLÅeuce e - Le Shelt.
Chemistry 12 Unit 2 - Chemical Equilibrium Chemistry 12 Worksheet 2-2 J LeChateliers PrinciIilie Name. In order to decide what effect a change in total pressure will have on an equilibrium system with gases what is the first thing you should do when given the balanced equation. Worksheet 111 KEY Molar Mass Calculations A mole is a standard unit of measurement for amount of a substance.
For an element one mole is equivalent to the mass of that element as it is listed on the periodic table and thus represents its molar mass. For a compound one must take the sum of all elements in the compound with. Formula Complete Net Ionic Equations Worksheet Key.